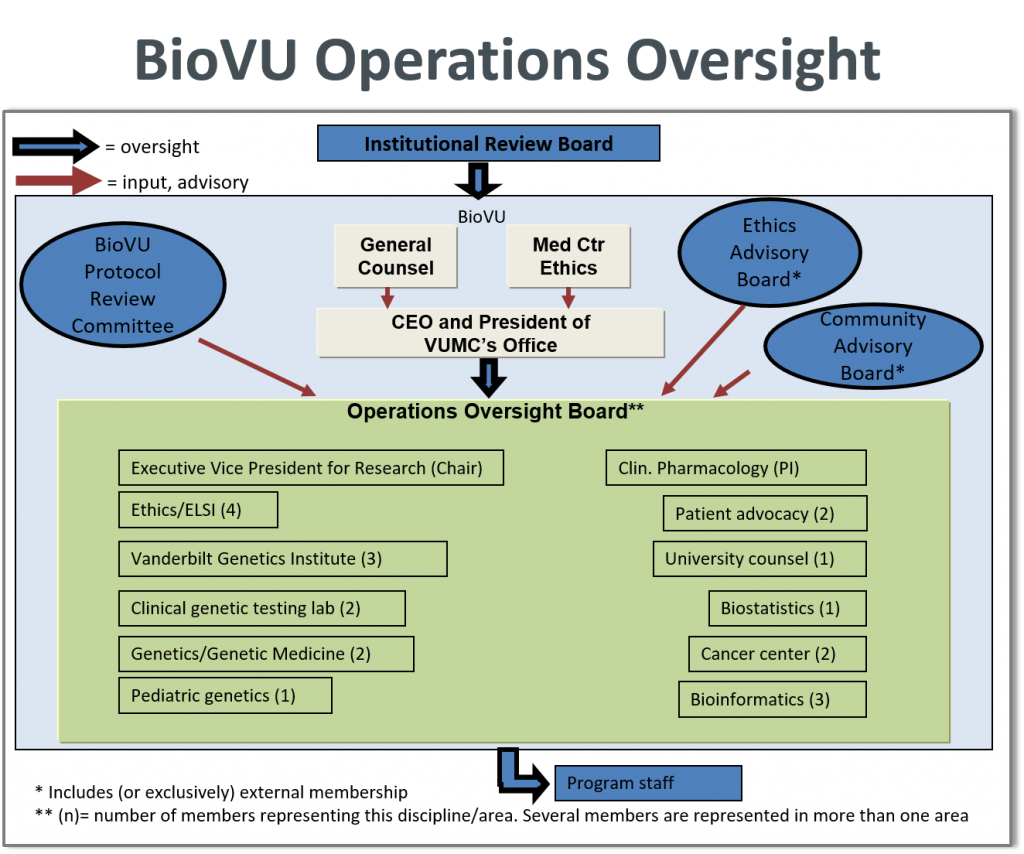
BioVU Oversight Boards
The Medical Center Ethics Committee (MCEC) was consulted during the planning phase of the BioVU biorepository and continues to provide oversight. Recommedations from the MCEC include:
- The Vanderbilt IRB will have on-going oversight
- All patients have the right to receive information about this project through a comprehensive education plan
- Patients have the right to refuse to participate by opting out of the program
- Advisory boards, which include external experts, should be established to provide oversight and guidance to BioVU
The Vanderbilt IRB will maintain oversight for the BioVU repository including all changes to the BioVU protocol including User Agreements, communication materials and protocols seeking to access the BioVU resource. The ultimate responsibility for compliance with regulations and policies rests with the Office of the CEO and President of Vanderbilt University Medical Center.
The BioVU Operations Oversight Board (OOB) guides BioVU operations. The OOB has been charged with guiding the implementation and accrual of the resource. The mission of the OOB is to maximize usefulness of the resource for investigators, assist in managing concerns and public challenges through education and communication, oversee core functions that enable the resource including de-identification and security, provide ongoing validation of the de-identification programming to ensure it meets current standards and continually evaluate the scientific goals of BioVU in light of financial, legal, ethical, social and practical constraints. The OOB incorporates feedback from the External Ethics Advisory Board (EAB) and the Community Advisory Board (CAB), described below.
Of particular importance in the oversight structure of BioVU is the presence of an External Ethics Advisory Board whose focus will be to evaluate the ethical issues arising from the development and usage of the repository. To address the ethical issues that may arise in the future related to the use and management of the repository, the EAB can be called on ad hoc for requests for opinions and consultation. They meet once per year in person for broader issues and project updates, and help the Institution address issues of broader impact.
BioVU also receives input from the Community Advisory Board (CAB), a centralized independent advisory body, which is made up of lay people active in the community. The mission is to ensure community involvement and input into the design and function of BioVU operations in order to evaluate, and ultimately support, acceptance among the broader medical and lay audiences The CAB provides opinions on issues of perceived social or ethics importance.
BioVU research proposals are required for investigators wishing to access BioVU samples or genotyping data. Research proposals are reviewed by the BioVU Review Committee which is composed of several members of the OOB, experts in genetics, biostatistics and epidemiology. Each proposal is reviewed for ethical concerns, feasibility of the project, benefit to the resource and scientific validity.